Hydrogen Basics:
+ What is hydrogen?
Hydrogen is the simplest, lightest and most abundant element in the universe making up more than 90% of all matter.
At room temperature, hydrogen forms a very light colourless gas and in daily life, when we refer to hydrogen, we actually refer to H2 or dihydrogen, which is the molecule made of two atoms of hydrogen usually in a gaseous form.
Hydrogen gas easily forms covalent compounds and therefore most of the Earth’s hydrogen exists in molecular compounds with non-metallic elements. Hydrogen is found in many widely used compounds, for example, bonded with oxygen it forms water (H2O) and hydrogen peroxide (H2O2), bonded with carbon it forms methane (CH4) and table sugar (C12H22O11), bonded with nitrogen it forms ammonia (NH3) and bonded with chlorine it forms hydrochloric acid (HCl). Hydrogen is also found in coal and oil and all living things as biomass.
The interest in hydrogen as an energy carrier comes from its high energy density at 120 MJ/kg, which is 2 to 4 times higher than coal (30 MJ/kg), petrol and diesel (42-46 MJ/kg) or natural gas (55 MJ/kg).
For more information on the physical and chemical properties of hydrogen please refer to Shell’s Hydrogen Study, Energy of the Future, Sustainable Mobility through Fuel Cells and H2 here.
+ Where do we get hydrogen from?
Hydrogen is the most abundant element in the universe and it can be extracted from all sorts of substances, including coal, oil, gas, biofuels, sewage sludge and water.
Existing technology that enables hydrogen to be extracted from coal, methane and water have long been proven and these allow the creation of hydrogen gas, which has been stored, transported and used for many decades.
The majority of hydrogen currently produced is made from fossil fuels by steam reforming of natural gas, partial oxidation of methane and coal gasification. Other methods of hydrogen production include biomass gasification and electrolysis of water. However, when hydrogen gas is made from the electrolysis of water powered by renewable electricity, no is CO2 is produced.
+ What is so good about hydrogen?
- Hydrogen is an energy carrier that can be made from renewable energy sources that produces only water when used. This makes it a clean zero emission fuel and as such it can play a vital role in advancing the energy transition away from polluting fossil fuels.
- Hydrogen is a high efficiency, low polluting fuel that can be used for transportation and stationary applications, particularly in places where it is difficult to use electricity.
- Hydrogen has the highest energy content of any common fuel by weight (3 times more energy dense than petrol or diesel).
- Hydrogen can be produced as a liquid or a gas and can be stored for long periods of time and transported over long distances, allowing the distribution of energy between regions and countries.
- Hydrogen is no more or less safe than petrol or diesel fuels.
- Hydrogen can provide low emission energy to all parts of the economy across the entire energy system including mobility and stationary applications such as heating, chemical feedstocks, large scale industrial processes and power generation.
+ What is hydrogen used for today?
Hydrogen is versatile and can be used in a wide range of applications. Click here to see a list of everyday uses for hydrogen. (click to our hydrogen uses link)
The multiple uses for hydrogen can be grouped into two large categories:
- Hydrogen as an industrial feedstock. Hydrogen has been used in a wide range of industrial processes for many decades and its use will continue to grow and also evolve as climate change obligations are prioritised by governments and organisations.
- Hydrogen as an energy carrier enabling the energy transition. The versatility of hydrogen, its ability to be used in a wide range of applications and that it can be produced and used with zero emissions, means that it has a role to play in decarbonising the economy.
Long established uses – hydrogen as an industrial feedstock
Supplying hydrogen to industrial users is a major global business. Demand for hydrogen has grown more than threefold in the past 40 years, with more than 70 million tonnes used around the world each year. This demand is expected to rise significantly and almost all of the industrial hydrogen feedstock used today is supplied from fossil fuels.
For more information on the International Energy Agency's future outlook of Hydrogen please refer to The Future of Hydrogen here
Most of the hydrogen produced today is from steam reforming of fossil fuels for use in several significant industrial processes.
Hydrogen is a fundamental building block for the manufacture of ammonia for fertiliser. It is also used to create a mixture of gases, such as synthesis gas, for fuel and for chemical feedstock for industrial processes, such as methanol and steel production, as well as for the refining intermediate oil products.
About 55% of the hydrogen produced around the world is used for ammonia synthesis, 25% in refineries and about 10% for methanol production. Other applications worldwide only account for about 10% of global hydrogen production.
For more information on what existing industries and uses of hydrogen please refer here
Enabling the energy transition – energy based uses
Hydrogen’s role in advancing the transition to a low emissions future is expanding rapidly as global investment across energy systems increases to unprecedented levels. The role that hydrogen can play across the energy system can be seen below:
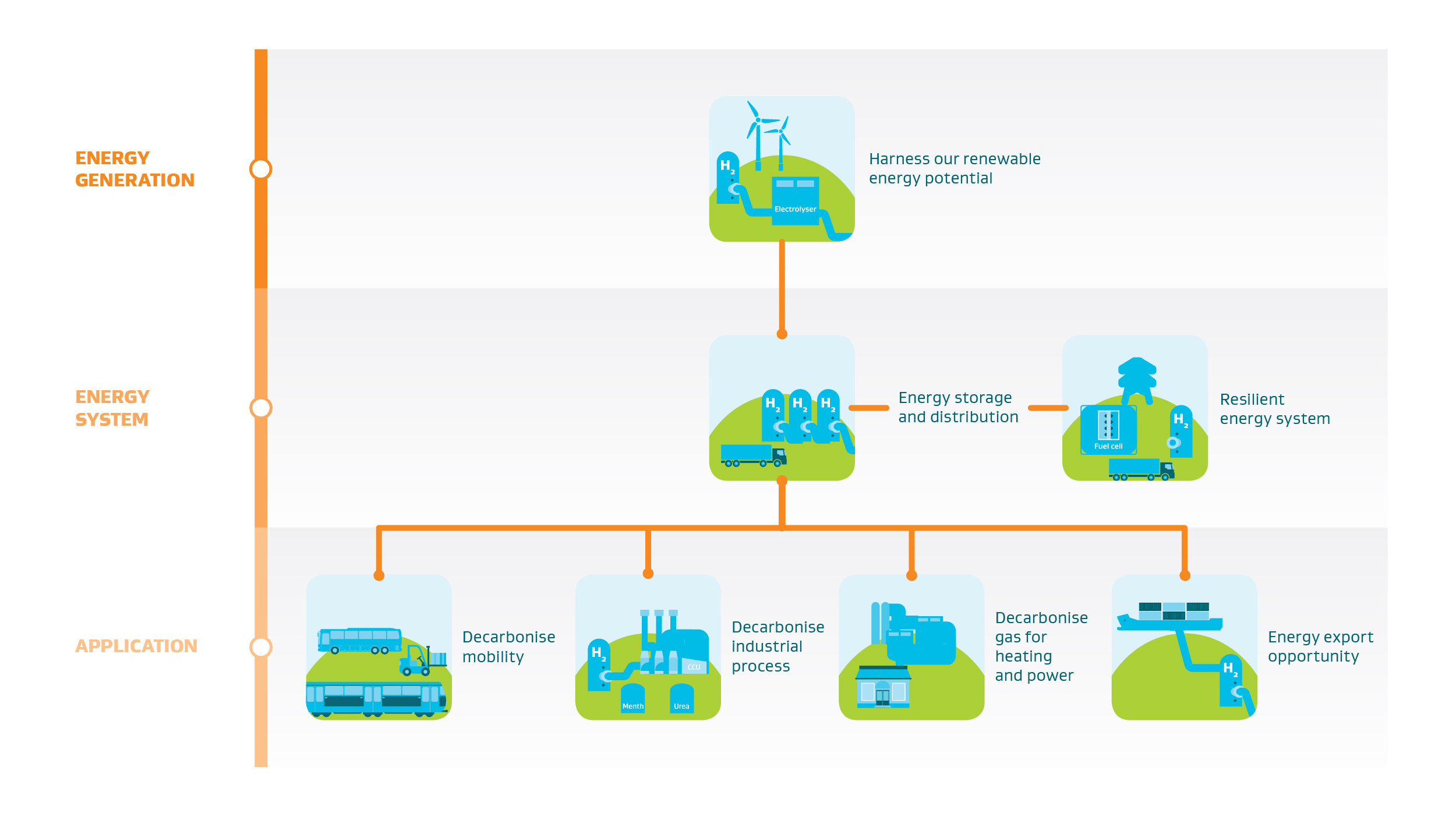 Reference & Acknowledgement: New Zealand Government Green Paper: A Vision for Hydrogen in New Zealand, Figure 3: Hydrogen's role in the New Zealand economy (p13). This map has been reproduced with kind permission provided by the Ministry of Business, Innovation and Employment (MBIE).
Reference & Acknowledgement: New Zealand Government Green Paper: A Vision for Hydrogen in New Zealand, Figure 3: Hydrogen's role in the New Zealand economy (p13). This map has been reproduced with kind permission provided by the Ministry of Business, Innovation and Employment (MBIE).
+ Does hydrogen have to be made from fossil fuels?
Currently, more than 99% of the hydrogen produced globally is produced from fossil fuels, with 6% of global natural gas production and 2% of global coal production used for the production of hydrogen. As a consequence, production of hydrogen is responsible for CO2 emissions of around 830 MtCO2/yr globally or the equivalent CO2 emissions of the UK and Indonesia combined.
For more information on the production of hydrogen globally please refer to the International Energy Agency's Hydrogen page here
Hydrogen does not have to be made from from fossil fuels. The technology to produce clean zero emissions hydrogen through the application of electricity to water (called electrolysis) is well established and has been used for more than 75 years. If the electricity used for the electrolysis process is from renewable sources, such as solar, wind or hydro the resulting hydrogen has zero carbon emissions.
Hydrogen can also be produced through biological reactions using microbes such as bacteria and microalgae. In these processes microbes consume biomass plant material and produce hydrogen gas.
For more information on hydrogen production please refer to Hydrogen Europe here
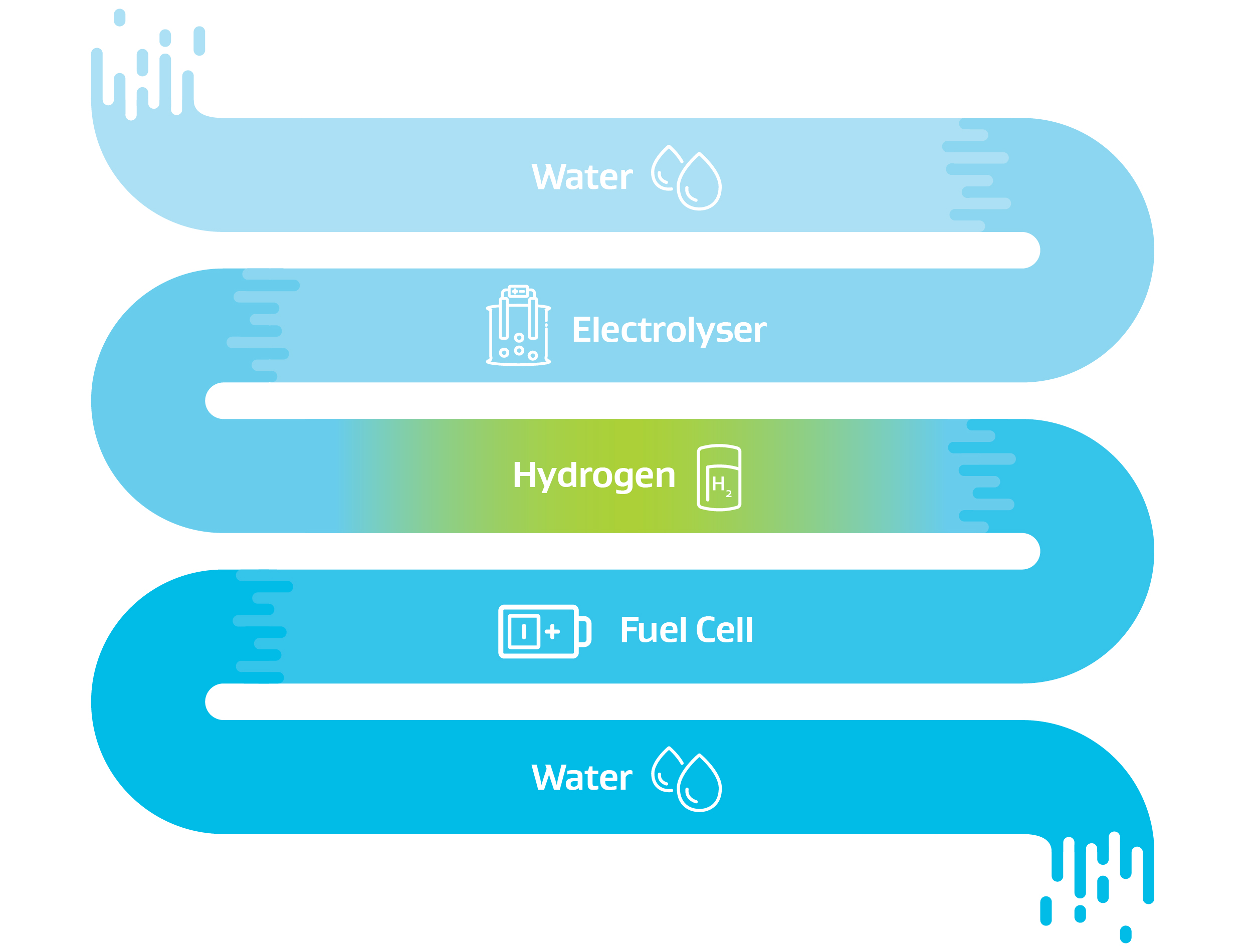 Reference & Acknowledgement: New Zealand Government Green Paper: A Vision for Hydrogen in New Zealand, Figure 4: Hydrogen Life Cycle - from water to water (p17). This diagram has been reproduced with kind permission provided by the Ministry of Business, Innovation and Employment (MBIE).
Reference & Acknowledgement: New Zealand Government Green Paper: A Vision for Hydrogen in New Zealand, Figure 4: Hydrogen Life Cycle - from water to water (p17). This diagram has been reproduced with kind permission provided by the Ministry of Business, Innovation and Employment (MBIE).
For more information on the use of hydrogen to decarbonise our economy please refer to the World Energy Council’s report Hydrogen – Industry as Catalyst, Accelerating the Decarbonisation of our Economy to 2030 here.
+ How much electricity and water is needed to make hydrogen?
To produce hydrogen via electrolysis at a refuelling station requires about 55 kWh/Kg H2 of electricity at an assumed rate of efficiency of >60% and 9 litres of water per Kg of hydrogen.
+ What are the different types of hydrogen?
Hydrogen can be produced, stored and converted through a range of different processes, which have been categorised and represented in simple colour terms as green, blue, grey and brown hydrogen. These colours are often used to depict the varying levels of carbon intensity of the different types of hydrogen produced.
- Green hydrogen is produced from renewable energy sources, most commonly through electrolysis, which splits water into hydrogen and oxygen. Green hydrogen does not use fossil fuels and produces zero or very low CO2.
- Blue hydrogen is produced from fossil fuels with the CO2 emissions produced captured and stored underground (CCS). Blue hydrogen is mainly derived from splitting natural gas into hydrogen and CO2 through steam methane reformation.
- Grey hydrogen is produced from industrial processes as a co-product.
- Brown hydrogen is produced from fossil fuels such as steam methane reformation of natural gas and coal gasification, and where the CO2 produced is released into the atmosphere.
Note: CCS technologies involve the capture of CO2 from fuel combustion or industrial processes; the transport of CO2 via ship or pipeline, and either its use as a resource to create valuable products or services ,or its permanent storage deep underground in geological formations.
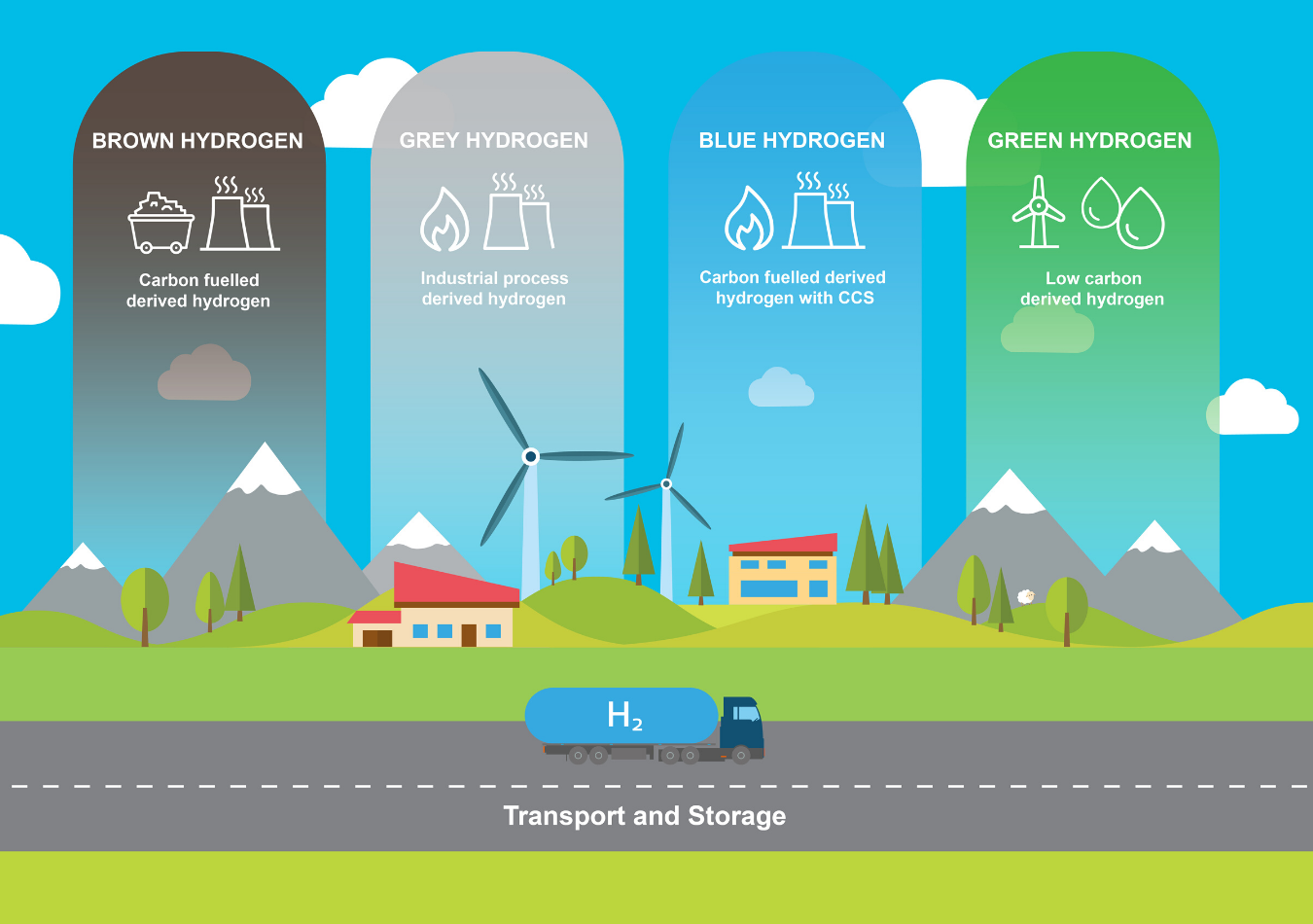 Reference & Acknowledgement: New Zealand Government Green Paper: A Vision for Hydrogen in New Zealand, Figure 15: Types of Hydrogen (p41). This image has been reproduced with kind permission provided by the Ministry of Business, Innovation and Employment (MBIE) and and Arup.
Reference & Acknowledgement: New Zealand Government Green Paper: A Vision for Hydrogen in New Zealand, Figure 15: Types of Hydrogen (p41). This image has been reproduced with kind permission provided by the Ministry of Business, Innovation and Employment (MBIE) and and Arup.
+ What is electrolysis?
Electrolysis is a process of using electricity to drive a chemical reaction to split water into its component parts of hydrogen and oxygen. The hydrogen is collected and the oxygen is either allowed to harmlessly escape or is also collected for use in a range of end use applications.
Electrolysis is an advanced, well understood process that has been used in various applications for over 150 years.
When the electricity used is from a renewable source, such as hydro, wind, solar, or geothermal, green hydrogen is produced, which when used in a fuel cell for example, produces zero emissions across the energy production and use lifecycle.
The electrolysis reaction takes place in a unit called an electrolyser. There are different types of electrolysers suitable for different applications. Electrolysers can range in size from small, appliance-size equipment that is well suited for small scale distributed hydrogen production, to large scale central production facilities linked directly to renewable electricity production and grids.
The greatest challenge for hydrogen production, particularly from renewable resources, is providing hydrogen at lower cost so that it becomes competitive with fossil fuel production. To reduce overall hydrogen cost, research is focused on improving the efficiency and lifetime of hydrogen production technologies as well as reducing the cost of capital equipment, operations, and maintenance.
Significant and unprecedently international investment is occurring in green hydrogen production and use technologies to decrease costs, increase efficiency and increase scale, all of which are expected to greatly improve.
For further informtion about the different types of electrolysers and how they work please refer to the Office of Energy Efficiency & Renewable Energy's Hydrogen Production: Electrolysis here.
+ What is an energy carrier?
An energy carrier is any system or substance that contains energy for conversion as useable energy later or somewhere else, such as the use of an electricity source from solar or wind that is used to convert water into hydrogen as an energy carrier for use in an appliance or fuel cell vehicle. Other energy carriers include petroleum, coal, wood, natural gas, electricity, electrical batteries, pressurised air and dammed water.
+ What is the difference between electricity and hydrogen as energy carriers?
Electricity and hydrogen are both energy carriers, however electricity is made up of electrons only and hydrogen is composed of molecules and electrons.
Hydrogen is energy dense and versatile and its molecular structure enables it to be stored for long periods of time, which provides the potential for it to replace fossil fuels. Electricity is more limited in its uses and in its storage capabilities.
There are energy efficiency losses with conversions between hydrogen’s physical and chemical forms, and these can be significant. While hydrogen’s energy efficiency losses are less than those from processing petrol and diesel, they do make hydrogen less efficient than electricity when we look at the full processing path to its end use.
Electricity and hydrogen have different operational advantages and disadvantages that are often complementary, not competing, and both energy carriers have an integrated role to play in the energy transition across our energy systems.
+ What is a fuel cell?
A fuel cell works like an electrolyser in reverse. It uses the chemical energy of hydrogen to cleanly and efficiently produce electricity, water and heat.
Fuel cells are unique in terms of the variety of their potential applications; they can provide power for systems as large as a utility power station and as small as a laptop computer, all potentially with zero emissions. Fuel cells are already being used for transportation such as cars, buses, scooters, trucks, marine vessels, aviation, material handling fork lift trucks as well as stationary, portable, and emergency backup power applications.
Fuel cells have several benefits over conventional combustion-based technologies currently used in many power plants and passenger vehicles. Fuel cells can operate at higher efficiencies than combustion engines and can convert the chemical energy in the hydrogen fuel to electrical energy with efficiencies of up to 60%. Fuel cells have lower emissions and fewer moving parts than combustion engines and when used with green hydrogen the fuel cell only emits droplets of water. Fuel cells are also quiet during operation, which makes them particularly suitable for indoor stationary uses and their zero emissions, quick refuelling and ability to operate in cold temperatures, makes them suitable for cold store materials handling facilities.
Learn more about the different types of fuel cells and how they work here
+ What can fuel cell’s be used for?
Fuel cells can play an important role in our national energy strategy across commercial, industrial and residential sectors. Hydrogen and fuel cells have the potential for use in a diverse range of applications including distributed or integrated combined-heat-and-power; backup power; systems for storing and enabling renewable energy; portable power; auxiliary power for trucks, aircraft, rail, and ships; specialty vehicles such as forklifts; and passenger and freight vehicles including cars, trucks, and buses.
Due to their high efficiency and zero, or near zero-emissions operation, hydrogen and fuel cells have the potential to reduce greenhouse gas emission in many applications.
For further information about hydrogen as an energy carrier see the Office of Energy Efficiency & Renewable Energy here
+ Is a fuel cell vehicle an electric vehicle?
Yes, the difference is that a fuel cell vehicle generates electricity as it moves through the chemical reaction with hydrogen. A battery electric vehicle carries all of its electricity in a battery.
Click here to see how a fuel cell car works.
+ What is Power-To-X?
Power-to-X describes methods for converting electrical energy (power) into other liquid or gaseous energy carriers or chemicals. In the hydrogen industry it involves the use of a renewable electricity current to split water via electrolysis to create green hydrogen for use in the energy transition. The X refers to the result of the conversion, which can be fuel, heat and/or chemicals.
Hydrogen Terminology:
Hydrogen Safety:
+ Is hydrogen safe?
For over 40 years, industry has used hydrogen in vast quantities as an industrial chemical and also as a fuel for space exploration. During that time, industry has developed extensive infrastructure to produce, store, transport and utilise hydrogen safely.
- Like gasoline and natural gas, hydrogen is flammable and can behave dangerously under specific conditions. As a result extensive international safety standards have been developed that enable the safe production, use, transport and storage of hydrogen that has grown to become a 70 Mt per year global industry and increasing.
- Hydrogen is no more or less dangerous than other flammable fuels, including gasoline and natural gas. In many cases, hydrogen is safer than the fuel we currently use to power our cars. Carbon-based fuels tend to spread as liquids and when they burn, conventional fuels produce hot ash, creating radiant heat. This isn't the case with hydrogen as in its pure form, hydrogen burns no carbon and produces no hot ash and very little radiant heat.
- Like any fuel, hydrogen has associated risks. Hydrogen is highly flammable, however when it leaks, as it is the lightest element in the world, it rapidly ascends into the atmosphere, which means it has limited time to burn. As a highly compressed gas, hydrogen requires clear rules of usage, exactly like as any other fuel.
- There are widespread misperceptions that spring from the absence of knowledge that hydrogen is already produced on an industrial scale and transported. It is used extensively around the world and can play a significant role in decarbonising industry and transport.
+ The Hindenburg: an incorrect perception
History has shown that public perceptions can be long-term negatively influenced by related historical incidents. A clear example of this is the Hindenburg disaster, which occurred in 1937 to which hydrogen was widely and wrongly attributed as the cause, and that perception still exists in the minds of people today. The Hindenburg disaster was the result of an electrical discharge from the clouds that occurred while docking during an electrical storm, which ignited the skin of the airship. The hydrogen burned quickly and safely above the occupants, but the diesel fuel onboard burned for up to ten hours after ignition. Unfortunately, the Hindenburg would have burned the same if it had been filled with inert helium gas, as the main cause of the Hindenburg accident was not the bags of hydrogen that provided the lifting force for the zeppelin, but a combination of the dark iron oxide and reflective aluminium paint that coated the surface of the ship. These components are extremely flammable and burn at a very high energetic rate once ignited.
+ Hydrogen and the H Bomb: nothing in common
Hydrogen is often confused with the “hydrogen bomb” which has led to the misperception that hydrogen as a fuel would transform a vehicle into a bomb on four wheels.
Hydrogen has three naturally occurring isotopes. The hydrogen industry uses the most common type of hydrogen: the stable isotope, Protium, which is the most prevalent hydrogen isotope, making up for more than 99.98% of all hydrogen in the universe.
Deuterium, which is found in the earth’s oceans, is the second most common form of stable hydrogen isotope and accounts for 0.02% of all hydrogen in the universe.
The hydrogen bomb technology uses the radioactive isotope called Tritium, which is extremely rare on earth (a billionth of a billionth of a %), with trace amounts formed by the interaction of the atmosphere with cosmic rays.
The hydrogen bomb requires Tritium plus the super-intense heat from the detonation of a nuclear fission bomb to induce the nuclear fusion reaction that makes a hydrogen bomb.
Tritium is radioactive and does not occur naturally, but can be made with lithium or a conventional nuclear reactor. This technology bears no resemblance to the simple chemical reactions associated with the Protium hydrogen isotope in hydrogen production, storage, distribution and uses in the hydrogen economy.
The International Association of Hydrogen Safety, Hysafe, is the focal point for all safety related issues of hydrogen.
Hydrogen in New Zealand:
+ Why is New Zealand considering hydrogen?
Hydrogen has the potential to provide economic benefits to New Zealand through export revenue, the creation of new industries and jobs, and as a zero emission fuel for use across the energy system where electrification is unachievable or prohibitive.
Green hydrogen has the potential to make a significant contribution to New Zealand achieving its climate change commitments of 100% renewable electricity by 2035 and zero net carbon emissions by 2050.
Hydrogen improves energy system resilience throughout the year, as once generated, hydrogen can be stored in both liquid and gaseous forms and used when required. Existing hydrogen technology can provide advantages over other energy carriers and many of its challenges are being actively addressed by significant international investment in research and development.
Globally, there is growing international interest in the development of a tradeable green hydrogen commodity market produced from renewable energy sources. Countries such as Japan and South Korea currently import most of their energy in the form of fossil fuels (coal, oil, natural gas), and are seeking to source clean energy solutions to meet their CO2 emission reduction targets. Green hydrogen is seen as having the potential to contribute to these goals. As yet, there are no large-scale exporters of hydrogen, presenting a significant economic opportunity for New Zealand.
+ What hydrogen projects are underway in New Zealand?
There are a range of hydrogen projects currently underlay in New Zealand, for further information regarding these see here
+ Which countries are leading in delivering hydrogen infrastructure?
Various European countries are focused on delivering an interconnected hydrogen refuelling network to facilitate the transition to zero emission mobility. In particular these include Germany, Belgium with regional refuelling infrastructure developing in the UK, Denmark, France and the Netherlands. Beyond Europe, Japan, South Korea and California are all investing heavily in delivering hydrogen technology.
See an H2Station's interactive map showing the refuelling infrastructure worldwide here.

![Contact 1. Inside Power station[44].jpg](https://images.squarespace-cdn.com/content/v1/5c350d6bcc8fedc9b21ec4c5/1702323694218-Q7JZK52MHBFHPLRDNCV3/Contact+1.+Inside++Power+station%5B44%5D.jpg)